Photoactivation of Adult-derived Adipose (Fat) Stem Cells
|
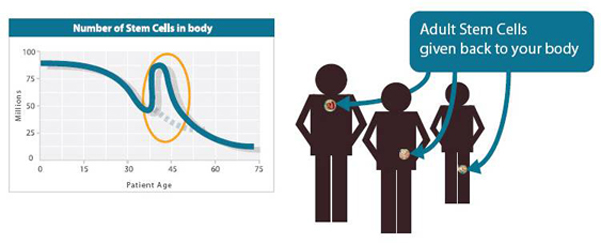 Adipose Tissue Yields an Abundance of ASC’s
Compared to any other source, the vast amounts of adipose tissue (depots of fat for storing energy) especially in the abdominal region, by sheer volume of availability, ensure an abundance in number of ASCs ranging in the millions per unit volume. The sheer number available also has the added advantage of not needing to be cultured in a laboratory over days in order to get the desired number of ASCs to achieve what is called “therapeutic threshold” i.e. therapeutic benefit. In addition, harvesting ASCs from adipose tissue through simple, minimally invasive liposuction under local anesthesia is relatively easier and painless — and poses minimal risk to the patient compared to all other possible methods.
Adipose tissue ASCs (AT-ASCs) are extremely similar to stem cells isolated from bone marrow (BMSCs). The similarities in profile between the two types of ASCs range from morphology to growth to transcriptional and cell surface phenotypes.4,5 Their similarity extends also to their developmental behavior both in vitro and in vivo. This has led to suggestions that adipose-derived stem cells are in fact a mesenchymal stem cell fraction present within adipose tissue.6
Clinically, however, stromal vascular fraction-derived AT-ASCs have the advantage over their bone marrow-derived counterparts, because of their abundance in numbers – eliminating the need for culturing over days to obtain a therapeutically viable number – and the ease of the harvest procedure itself – being less painful than the harvest of bone marrow. This, in theory, means that an autologous transplant of adipose-derived ASCs will not only work in much the same way as the successes shown using marrow-derived mesenchymal stem cell transplant, but also be of minimal risk to the patient.
AT-ASCs, like BM-ASCs, are called Mesenchymal ASCs because they are both of mesodermal germ-origin. This means that AT-ASCs are able to differentiate into specialized cells of mesodermal origin such as adipocytes, fibroblasts, myocytes, osteocytes and chondrocytes.7,8,9 AT-ASCs are also able (given the right conditions of growth factors) to transdifferentiate into cells of germ-origin other than their own. Animal model and human studies have shown AT-ASCs to undergo cardiomyogenic 10, endothelial (vascular)11, pancreatic (endocrine) 12, neurogenic 13, and hepatic trans-differentiation14 , while also supporting haematopoesis15.
Low Risk to the Patient
Adipose Tissue Yields an Abundance of ASC’s
Compared to any other source, the vast amounts of adipose tissue (depots of fat for storing energy) especially in the abdominal region, by sheer volume of availability, ensure an abundance in number of ASCs ranging in the millions per unit volume. The sheer number available also has the added advantage of not needing to be cultured in a laboratory over days in order to get the desired number of ASCs to achieve what is called “therapeutic threshold” i.e. therapeutic benefit. In addition, harvesting ASCs from adipose tissue through simple, minimally invasive liposuction under local anesthesia is relatively easier and painless — and poses minimal risk to the patient compared to all other possible methods.
Adipose tissue ASCs (AT-ASCs) are extremely similar to stem cells isolated from bone marrow (BMSCs). The similarities in profile between the two types of ASCs range from morphology to growth to transcriptional and cell surface phenotypes.4,5 Their similarity extends also to their developmental behavior both in vitro and in vivo. This has led to suggestions that adipose-derived stem cells are in fact a mesenchymal stem cell fraction present within adipose tissue.6
Clinically, however, stromal vascular fraction-derived AT-ASCs have the advantage over their bone marrow-derived counterparts, because of their abundance in numbers – eliminating the need for culturing over days to obtain a therapeutically viable number – and the ease of the harvest procedure itself – being less painful than the harvest of bone marrow. This, in theory, means that an autologous transplant of adipose-derived ASCs will not only work in much the same way as the successes shown using marrow-derived mesenchymal stem cell transplant, but also be of minimal risk to the patient.
AT-ASCs, like BM-ASCs, are called Mesenchymal ASCs because they are both of mesodermal germ-origin. This means that AT-ASCs are able to differentiate into specialized cells of mesodermal origin such as adipocytes, fibroblasts, myocytes, osteocytes and chondrocytes.7,8,9 AT-ASCs are also able (given the right conditions of growth factors) to transdifferentiate into cells of germ-origin other than their own. Animal model and human studies have shown AT-ASCs to undergo cardiomyogenic 10, endothelial (vascular)11, pancreatic (endocrine) 12, neurogenic 13, and hepatic trans-differentiation14 , while also supporting haematopoesis15.
Low Risk to the Patient
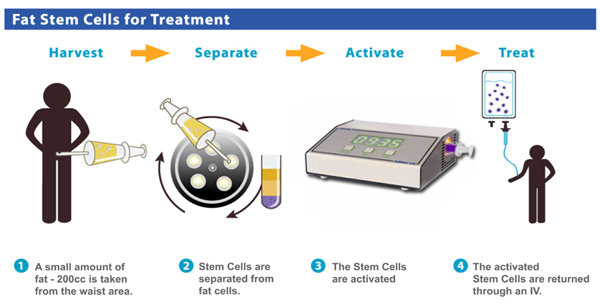 Autologous transplant of SVF AT-ASCs also poses extremely low risk to the patient when done as a single procedure in a sterile surgical operating room setting. Furthermore, it is postulated that SVF AT-ASCs due to their immunosuppressive properties may be transplanted into not only autologous but also allogenic tissues without initiating a cytotoxic T-cell response.16 We believe that autologous transplant is the safest and most viable option at this point.
It is noteworthy that the protocol for the procurement of SVF AT-ASCs does not overlook the therapeutic potential conferred by the cocktail of ingredients present in the SVF. Let us look at this cocktail of cells, proteins and growth factors in a little more detail.
The extracellular matrix of adipose tissue contains different types of Collagen such as Types 1, 3-4, 7, 14-15, 18 and 27 to name a few.6 This is important in the Fat Transfer protocol where freshly isolated fat is used as a filler in augmentation or post-lumpectomy reconstruction of the breast, augmentation of the penis, reconstruction of the arthritic joints, and where collagen provides the structural support required for cell survival.
Furthermore, the extracellular matrix plays an important role in adipocyte endocrine secretions, and release of growth factors such as transforming growth factor beta (TGF-ß), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF), among others all of which are contained in the SVF.17 This is consistent with the secretions of cells in the presence of an extracellular matrix. The SVF also contains the various proteins present in the adipose tissue extracellular matrix of which laminin is of interest due to its ability to help in neural regeneration.6
The cellular composition of the SVF ranges from pre-adipocytes to endothelial cells, smooth muscle cells, pericytes, fibroblasts, and AT-ASCs. Typically, the SVF also contains blood cells from the capillaries supplying the fat cells. These include erythrocytes, B and T cells, macrophages, monocytes, mast cells, natural killer (NK) cells, hematopoietic stem cells and endothelial progenitor cells, to name a few. The latter two types of cells, namely the hematopoietic stem cells and endothelial progenitor cells play important roles in supporting the viability of existing blood vessels and helping create new ones respectively.
We believe that these other ingredients that make up the SVF ‘cocktail’ act as an adjuvant to further augment the effect of the autologous transplant of SVF AT-ASCs.
Autologous transplant of SVF AT-ASCs also poses extremely low risk to the patient when done as a single procedure in a sterile surgical operating room setting. Furthermore, it is postulated that SVF AT-ASCs due to their immunosuppressive properties may be transplanted into not only autologous but also allogenic tissues without initiating a cytotoxic T-cell response.16 We believe that autologous transplant is the safest and most viable option at this point.
It is noteworthy that the protocol for the procurement of SVF AT-ASCs does not overlook the therapeutic potential conferred by the cocktail of ingredients present in the SVF. Let us look at this cocktail of cells, proteins and growth factors in a little more detail.
The extracellular matrix of adipose tissue contains different types of Collagen such as Types 1, 3-4, 7, 14-15, 18 and 27 to name a few.6 This is important in the Fat Transfer protocol where freshly isolated fat is used as a filler in augmentation or post-lumpectomy reconstruction of the breast, augmentation of the penis, reconstruction of the arthritic joints, and where collagen provides the structural support required for cell survival.
Furthermore, the extracellular matrix plays an important role in adipocyte endocrine secretions, and release of growth factors such as transforming growth factor beta (TGF-ß), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF), among others all of which are contained in the SVF.17 This is consistent with the secretions of cells in the presence of an extracellular matrix. The SVF also contains the various proteins present in the adipose tissue extracellular matrix of which laminin is of interest due to its ability to help in neural regeneration.6
The cellular composition of the SVF ranges from pre-adipocytes to endothelial cells, smooth muscle cells, pericytes, fibroblasts, and AT-ASCs. Typically, the SVF also contains blood cells from the capillaries supplying the fat cells. These include erythrocytes, B and T cells, macrophages, monocytes, mast cells, natural killer (NK) cells, hematopoietic stem cells and endothelial progenitor cells, to name a few. The latter two types of cells, namely the hematopoietic stem cells and endothelial progenitor cells play important roles in supporting the viability of existing blood vessels and helping create new ones respectively.
We believe that these other ingredients that make up the SVF ‘cocktail’ act as an adjuvant to further augment the effect of the autologous transplant of SVF AT-ASCs.
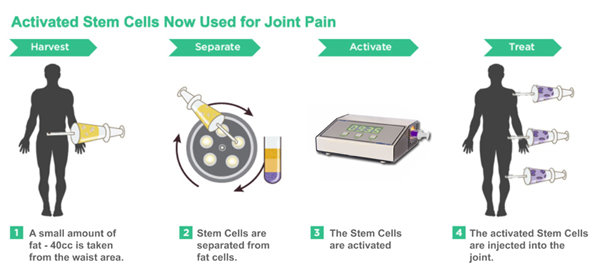
Using ASCs for Stem Cell Treatment with Photoactivation for Joint Pain
Stem Cell Expansion is Unnecessary
An important point to note is that there is still debate whether freshly isolated ASCs are functionally similar to ASCs which have undergone expansion.18 We believe this debate to be of little consequence because of the vast numbers of ASCs we are able to harvest — and expansion is therefore unnecessary. Moreover, our own preliminary results in human subjects (n=32), where wound-healing was tested by the introduction of freshly isolated ASCs into the wound showed more than promising results. It must be stated however, that isolates from the lipoaspirate on its own proved less effective than when the isolates were introduced into either a proprietary Activation Medium containing known growth factor stimulators of stem cells in addition to the patients’ own platelet-derived growth factors (using PRP Kit) for one hour before being re-introduced into the patient.
ASCs Require Activation for Full Functionality
The observations stated above, confirms the theory that Adipose-derived ASCs though large in number lie dormant within the adipose tissue and that they require activation to come into full functionality for more successful implantation into the host tissue and to begin self-renewal by cell division and formation of other cell types by differentiation and transdifferentiation. This is also in line with the theory that ASCs are called into action only when the tissues within which they reside are dying, damaged or diseased. Further preliminary testing to increase the functionality of the Adipose-derived ASCs using specific frequencies of monochromatic light has also revealed promising results.
Clinical Trials in Humans on the Safety and Efficacy of Administration of Activated Autologous Adipose-derived Stromal Vascular Fraction Adult Stem Cells are completed at various centers around the world for Management of Type II Diabetes, Breast Reconstruction Post-Lumpectomy, Management and Healing of Chronic Diabetic Ulcers and for Idiopathic Pulmonary Fibrosis.
Future research areas which have shown promising results in our initial case studies are Osteoarthritis, Emphysema, Stroke, Heart Failure and early stage Parkinson’s Disease.
Stem Cells and PRP
Wound healing is a complex process, involving a mechanism of complex cascading regulatory events at both the molecular and cellular levels.19,20 Growth factors (GFs) are secreted by a wide variety of cells to regulate the wound healing process in an orderly manner.21,22 Over the last decade, various GFs, including platelet-derived growth factor (PDGF), and transforming growth factor-beta (TGF-ß), have been used to accelerate the healing process.2327
Platelet-rich plasma (PRP), as a storage vehicle of growth factors, is a new application of tissue engineering which was considered for the application of growth factors. PRP is a concentration of platelets in plasma developed by gradient density centrifugation.28 It contains many growth factors, such as PDGF, TGF-ß, vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), insulin-like growth factor (IGF), etc.29,30 And it has been successfully used in a variety of clinical applications for improving hard and soft tissue healing.3135 Platelet-rich plasma has also been shown to enhance the proliferation of human adipose-derived stem cells.36
The (stem cell) procedure involves the taking of blood during or just prior to the patient’s adipose tissue extraction procedure. Platelets are isolated from the blood and then activated to release their growth factors before photoactivation. The adipose-derived ASCs are then mixed with the growth factors containing plasma and activated in the Adi-Light 2 for 20 minutes prior to being administered to the patient.
References
1 Filip S, English D and Mokry J (2004). Issues in stem cell plasticity. J Cell Mol Med 8 (4): 572-577.
2 Filip S, Mokrý J, Hruška I (2003) Adult stem cells and their importance in cell therapy. Folia Biol.(Prague) 49: 9-14.
3 Gimble JM, Katz AJ, Bunnell BA (2007) Adipose-derived Stem Cells for Regenerative Medicine Circ Res. 100:1249-1260.
4 Katz AJ, Tholpady A, Tholpady SS, et al. (2005) Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells 23(3):412-23.
5 Pittenger MF, Martin BJ. (2004) Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 95(1):9-20.
6 Tholpady SS, Llull R, Ogle RC, et al. ((2006) Adipose Tissue: Stem Cells and Beyond. Clin Plastic Surg 33:55-62
7 Zuk PA, Zhu M, Mizuno H, Huang JI, Chaudhari S, Lorenz HP, Benhaim P and Hedrick MH (2001). “Mutilineage cells derived from human adipose tissue: a putative source of stem cells for tissue engineering”. Tissue Engineering 7 (2): 211-216.
8 Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P and Hedrick MH (2002). “Human adipose tissue is a source of multipotent stem cells”. Mol Biol Cell 13: 4279-4295.
9 Mizuno H, Zuk PA, Zhu M, et al. (2002) Myogenic differentiation by human processed lipoaspirate cells. Plast Reconstr Sur 109:199-209.
10 Planat-Benard V, Menard C, Andre M, et al. (2004) Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res 94:223-229.
11 Cao Y, Sun Z, Liao L, et al. (2005) Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun 332:370-379.
12 Timper K, Seboek D, Eberhardt M, et al. (2006) Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem Biophys Res Commun 341:1135-1140.
13 Safford KM, Hicok KC, Safford SD, et al. (2002) Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun 2002;294:371-379.
14 Seo MJ, Suh SY, Bae YC et al. (2005) Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun 328:258-264.
15 Corre J, Barreau C, Cousin B, et al. (2006) Human subcutaneous adipose cells support complete differentiation but not self-renewal of hemato-poietic progenitors. J Cell Physiol 208:282-288.
16 Yanez R, Lamana ML, Garcia-Castro J, et al.(2006) Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells 24:2582-2591.
17 Nakagami H, Morishita R, Maeda K, et al. (2006) Adipose Tissue-Derived Stromal Cells as a Novel Option for Regenerative Cell Therapy. J Atheroscler Thromb 13:77-81.
18 Miranville A, Heeschen C, Sengenes C, et al. (2004) Improvement of postnatal neovascularization by human adipose issue-derived stem cells. Circulation;110(3):349-55.
19 Conway EM, Collen D, Carmeliet P. (2001) Molecular mechanisms of blood vessel growth. Cardiovasc Res 49: 507-521.
20 Koveker GB. (2000) Growth factors in clinical practice. Int J Clin Pract 54: 590-593.
21 Murakami S, Takayama S, Ikezawa K, Shimabukuro Y, Kitamura M, Nozaki T, et al. (1999) Regeneration of periodontal tissues by basic fibroblast growth factor. J Periodontol Res 34: 425-430.
22 Murakami S, Takayama S, Ikezawa K, Shimabukuro Y, Kitamura M, Nozaki T. (2000) Bone growth factors. Orthop Clin North Am 31: 375-388.
23 Stefani CM, Machado MA, Sallum EA, Sallum AW, Toledo S, Nociti FH Jr. (2000) Platelet-derived growth factor/insulin-like growth factor-1 combination and bone regeneration around implants placed into extraction sockets: a histometric study in dogs. Implant Dent 9: 126-31.
24 Safford KM, Hicok KC, Safford SD, et al. (2002) Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun 2002;294:371-379.
25 Vuola J, Bohling T, Goransson H, Puolakkainen P. (2002) Transforming growth factor beta released from natural coral implant enhances bone growth at calvarium of mature rat. J Biomed Mater Res 59: 152-159.
26 Jiang D, Dziak R, Lynch SE, Stephan EB. (1999) Modification of an osteoconductive anorganic bovine bone mineral matrix with growth factors. J Periodontol 1999; 70: 834-839.
27 Sun Y, Zhang W, Ma F, Chen W, Hou S. (1997) Evaluation of transforming growth factor beta and bone morphogenetic protein composite on healing of bone defects. Chin Med J; 110: 927-31.
28 Whitman DH, Berry RL, Green DM. (1997) Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg 1997; 55: 1294-1299.
29 Weibrich G, Kleis WK, Hafner G, Hitzler WE. (2002) Growth factor levels in platelet-rich plasma and correlations with donor age, sex and platelet count. J Craniomaxillofac Surg; 30: 97-102.
30 Landesberg R, Roy M, Glickman RS. (2000) Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg; 58: 297-300.
31 Ouyang XY, Qiao J. (2006) Effect of platelet-rich plasma in the treatment of periodontal intrabony defects in humans. Chin Med J; 119: 1511-1521.
32 Nikolidakis D, van den Dolder J, Wolke JG, Jansen JA. (2008) Effect of platelet-rich plasma on the early bone formation around Ca-P-coated and non-coated oral implants in cortical bone. Clin Oral Implants Res 19: 207-213.
33 Schaaf H, Streckbein P, Lendeckel S, Heidinger K, Görtz B, Bein G, et al. (2008) Topical use of platelet-rich plasma to influence bone volume in maxillary augmentation: a prospective randomized trial.Vox Sang 94: 64-69.
34 Chang T, Liu Q, Marino V, Bartold PM. (2007) Attachment of periodontal fibroblasts to barrier membranes coated with platelet-rich plasma. Aust Dent J 52: 227-233.
35 Kitoh H, Kitakoji T, Tsuchiya H, Katoh M, Ishiguro N. (2007) Distraction osteogenesis of the lower extremity in patients with achondroplasia/hypochondroplasia treated with transplantation of culture-expanded bone marrow cells and platelet-rich plasma. J Pediatr Orthop 27: 629-634.
36 Kakudo N, Minakata T, Mitsui T, Kushida S, Notodihardjo FZ, Kusumoto K. (2008) Proliferation-promoting effect of platelet-rich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plast Reconstr Surg. Nov122(5):1352-60.

Recent Comments